Back in July 2017, Kolon Life Science’s drug Invossa became the country’s first gene therapy to gain approval from the Ministry of Food and Drug Safety (MFDS). The Invossa drug was a cell-based therapy for osteoarthritis. It was later found to be GP2-293 cells derived from kidneys, not cartilage cells! Although the marketing authorization was revoked in July 2019, in the space of just 2 years, Invossa was administered to a total of 3,235 individuals, including 240 clinical trial participants and 2,995 post-market patients. Beyond the horrendous cost of mislabeling and false reporting of ingredients, the Korean government had to repay $39.1 million to Mitsubishi Pharma. The FDA suspended patient enrollment in the Phase III clinical trial (subsequently lifted).
Beyond the pure financial cost, Invossa has been linked to numerous reported cases of tumors in patients after administration. Of the 90 tumor-related cases, with the causal relationship with Invossa was classified as difficult to evaluate in 73 cases and impossible to evaluate in 17 cases. Still, as of August 2023, 2,812 patients who received Invossa have registered in the ministry’s long-term tracking system.
All this would have been avoidable with an approximately 100 dollar STR test!
Kolon Life Science’s Invossa
Have we really learned from other people’s mistakes?
According to the ICLAC Register of Misidentified Cell Lines, as of April 2024, there are still:
- 545 misidentified cell lines with no known authentic stock,
- 78 that do not correspond to the original donor but the contaminant is unknown,
- 70 cell lines coming from a different species (interspecies contamination), and 9 cell lines coming from a non-human species where intraspecies contamination has been detected,
- 157 different contaminants listed. The most common is HeLa (145 entries), followed by T-24 (21 entries) and M14 (18 entries).

Particular caution should be exercised if your cell line is from a secondary source (e.g. the neighboring laboratory), as it will be twice as likely to be misidentified than if your cell line source was obtained from a primary source, such as the investigators who established the cell line or from a certified cell line bank. Indeed, a retrospective study from the German Collection of Microorganisms and Cell Culture (DSMZ) (Drexler et al., 2017) including 848 leukemia-lymphoma cell lines received between 1990 and 2014 indicated that 1 in 6 of the secondarily sourced cell lines, shared between laboratories, is misidentified.
How did we let it get to this point?
All labs big and small are confronted with this issue, which is more prevalent in R&D where the chances of cross-contamination between lines are higher than in GMP environments. In the very engaging video “The hidden world of cell lines”, ICLAC very effectively demonstrates how easily a lab can end up with a misidentified cell line. Watch video
Despite the seriousness of the issue, the main resistance seems to come from scientists trying to evade the truth. University of Colorado genetics Christopher Korch explains this well in the article “Line of attack” published in Science in February 2015. According to Korch, even after finding out that the cell line they have been working on is not what they thought it was, some scientists believe it is still a valid model because its behavior seems to match their expectations.
Other scientists face the issue of scarcity of specific cell lines that they need to use for their research, as is the case for RGC-5, which has been wrongly used as a model for retinal ganglion cells.
Whatever the reason, continuing to use misidentified cell lines is counter-productive, weakens research and slows down discoveries.
No budget for testing… really?
It is common knowledge that when budgets are tight, this can lead to cutting-corners, often at the expense of a thorough QC. However, when we pause and think for a second and look at the cost of your overall research (cost of media, premises, staff, etc.) one can argue that if you cannot afford to authenticate your cell lines, you likely cannot afford to do your research at all. Particularly if the cost of working on an impostor cell line translates to exponential loss of revenue!
Experimental pathologist John Masters of University College London and ICLAC member estimates that $100,000 is the typical cost of an average cell study. Korch estimated that$713 million has been spent on published work involving just HEp-2 and INT 407 (two famously wrongly identified cell lines). And that is only on just 2 lines! Imagine what the amount could be for the 545 impostor cell lines in the ICLAC database!
Of course, this can go further and affect human lives, as it was clearly demonstrated in the real-life Invossa case study.
Towards a brighter future?
ICLAC estimates that as many as 1/5 to 1/3 of known cell lines are still wrongly identified to date (2021 data). However, there is now awareness of the issue, simple and affordable testing solutions available to all labs, new reporting practices for publishing results and pre-requisites when applying for NIH funding… ICLAC even provides support in training scientists who are new to the topic.
All in all, stem cell scientists around the world can now have access to clear guidelines. The field owes a lot to the likes of Stan Gartler, Walter Nelson-Rees, Roland Nardone or Christopher Kirch, who have gone on a long crusade to conquer the issue of cell line authentication. All their combined work is translating into significant inroads in shaping mentalities towards more rigor and reproducibility in stem cell research.
We are on the right track!
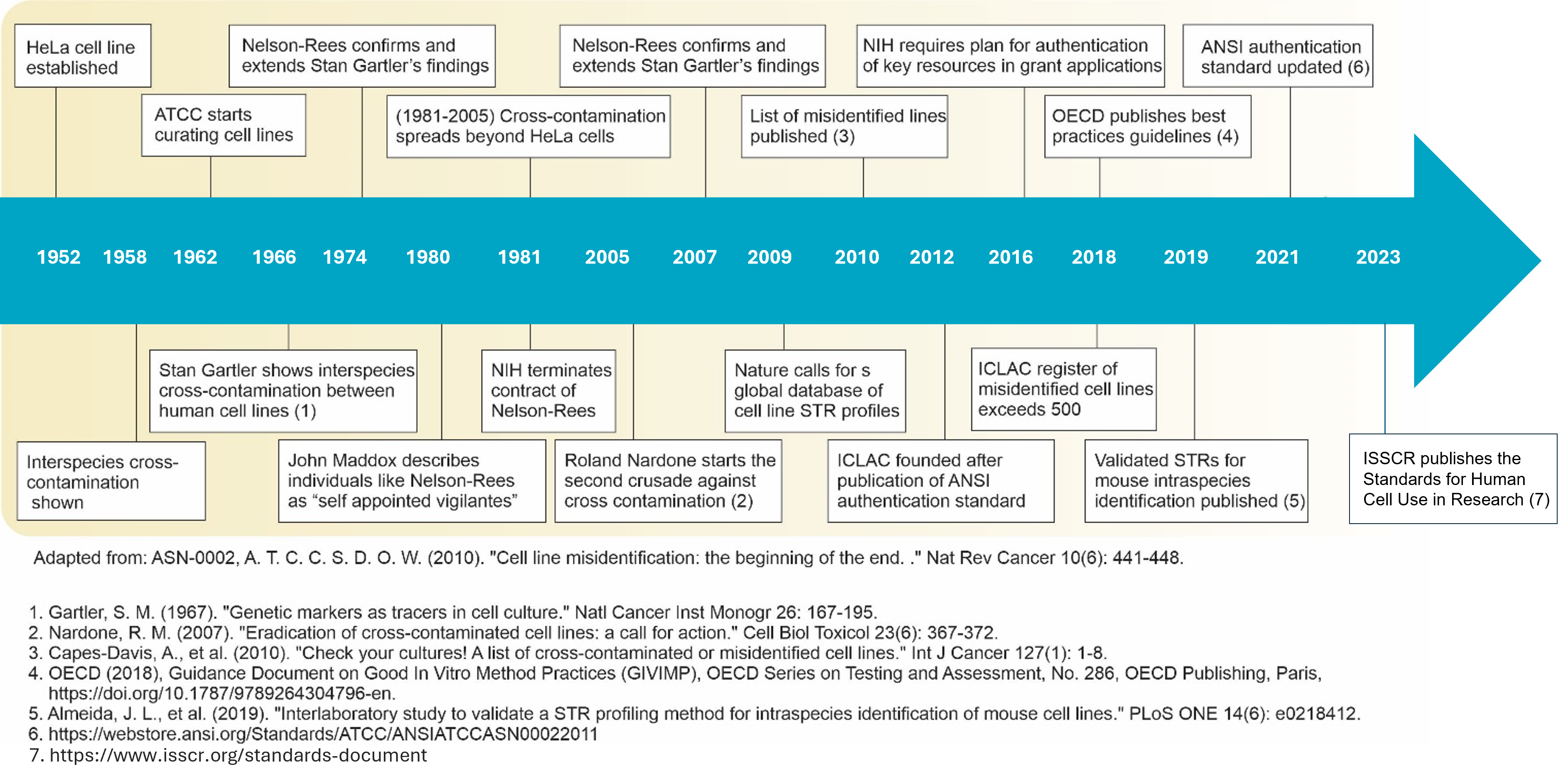
Source: adapted from the ICLA website. A history of addressing cell line misidentification
Key milestones:
- ANSI Standard (ASN-0002): Authentication of Human Cell Lines: Standardization of STR Profiling was published in 2012
- Numerous journals and granting agencies are now requiring authentication of cell lines prior to publication and funding
- The FDA has instituted a requirement for the authentication of cell lines used to produce pharmaceuticals 21 CFR211.160 (b) 21 CFR 610.18 (b)
- NIH has revised guidelines to applications for funding and provided guidelines for reporting
- Enhanced Reproducibility through Rigor and Transparency (effective Jan. 25, 2016) Notice Number: NOT-OD-15-103
- NIH Rigor and Reproducibility: Principles and Guidelines for Reporting Preclinical Research and Endorsement by major journals
- In June 2023, the ISSCR published The Standards for Human Stem Cell use in Research for more reproducibility and rigor in research. Cell authentication takes up a large part of the document that also offers new reporting practices for publishing results (checklist).
To go further…
Many commercial suppliers (biotech, core facilities, etc.) can run the test for you, but if you want to deepen your understanding on the topic, the ICLAC website is a must-read. It is packed with first-class information and useful advice for scientists. You can have free access to training sessions, check-lists and guides, so cell line authentication will not hold any more secrets for you!
References:
- The International Cell Line Authentication Committee (ICLAC) website: https://iclac.org/
- Drexler, H. G., Dirks, W. G., MacLeod, R. A. F., & Uphoff, C. C. (2017). False and mycoplasma-contaminated leukemia–lymphoma cell lines: time for a reappraisal. International Journal of Cancer, 140(5), 1209–1214. https://doi.org/10.1002/ijc.30530
- Hurst, J., Attrodt, G., Bartz-Schmidt, K. U., Mau-Holzmann, U. A., Spitzer, M. S., & Schnichels, S. (2023). A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?” International Journal of Molecular Sciences, 24(18). https://doi.org/10.3390/ijms241813801
- Korea Biomedical Review, article “Kolon Life Science’s gene therapy Invossa linked to 90 tumor cases: regulator”, Lee Han-soo, Published 2023.09.18
- Science, article “Line of attack”, Jill Neimark, Published 2015.02.27
- The Scientist, article “The Great Big Clean-Up”, Kerry Grens, Published 2015.09.01
- The ISSCR Standards for Human Stem Cell Use in Research, June 2023. Downloadable here.

