
Téléthon 2025: Let’s support research to find cures !
Stem Genomics is joining the Telethon’s fight against neuromuscular diseases.

Stem Genomics is joining the Telethon’s fight against neuromuscular diseases.

Read about Stem Genomics social responsibility inititiaves in the sustainability of local rural
Stem Genomics announces the launch of Pluri-digital™, a new pluripotency assay that is

iPSC research is moving fast towards a breakthrough, and this has a direct

Read about the latest design upgrade of our flagship genomic stability assay,

Watch a short video for a visual presentation of our latest NGS-targeted assay

Dr R. MARCU explains why she finds the Stem-Seq™ Plus test relevant in

Up to 5 essential tests performed on the same sample in record-breaking time.

Pluristyx, Inc. and Stem Genomics SAS today announce the closing of an equity
Find out about our latest NGS-based genomic stability assay, specifically designed for stem

You can now order our genomic stability services from our US-based laboratory located

When it comes to assessing a genomic stability assay, do repeatability, mosaicism, false
Stem Genomics introduces Duo iCS-Karyo: iCS-digital™ PSC and G-Banding karyotype combined analysis. Watch

Big milestone for the team here at Stem Genomics: we have received the

Many of you asked for a test that would focus on the most
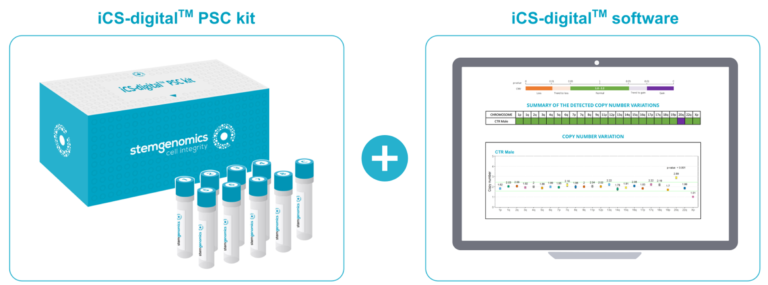
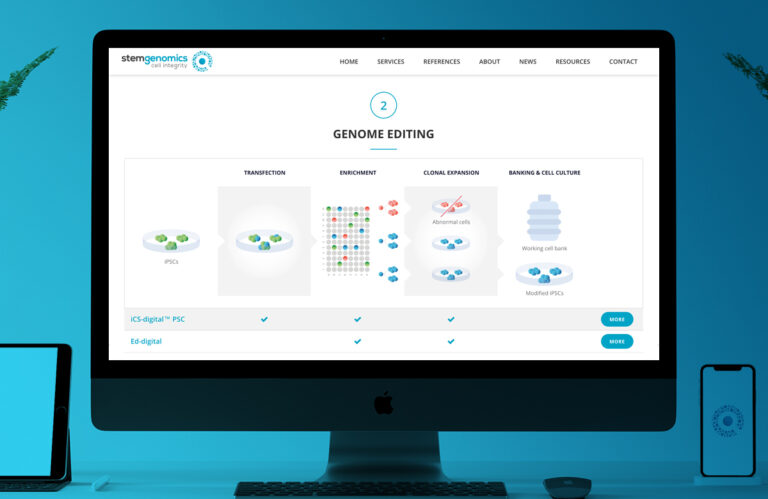
Stem Genomics diversifies its offer by launching the iCS-digital TM PSC kit…
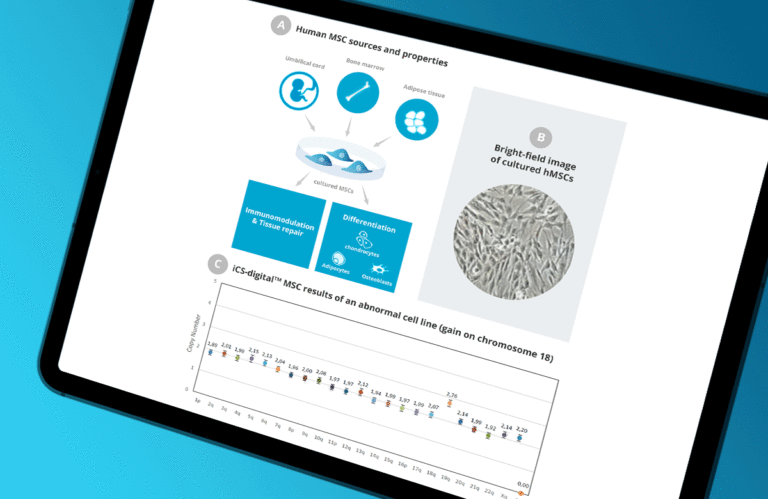
The iCS-digital™ MSC test is a straightforward method for the routine monitoring of
Recurrent Genetic Abnormalities in Human Pluripotent Stem Cells: Definition and Routine Detection in
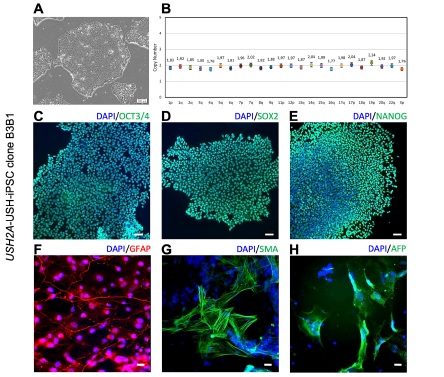
Genome Editing in Patient iPSCs Corrects the Most Prevalent USH2A Mutations and Reveals

Recurrent genetic abnormalities in human pluripotent stem cells: definition and routine detection in
The iCS-digital™ PSC test offers fast and reliable detection of the most common

iCS-digital™ PSC: A method for monitoring hPSC genome integrity that detects more than

The iCS-digital™ PSC test was used to evaluate the genomic integrity of hiPSCs

We are quick to respond
Let's talk to each other
According to your availability
Subscribe to stay informed
Cap Sigma
1682 rue de la Valsière
34 790 Grabels
400 Park Offices Drive
Room 105
Research Triangle Park
Durham, NC 27713

By continuing your browsing, you agree to the use of cookies for statistical and personalization purposes. The cookies collect information in a way that does not directly identify anyone. We respect your privacy, here's how
As of July 17, our new address will be:
400 Park Offices Drive, Room 105
Research Triangle Park, Durham, North Carolina 27713
Please note that we will be closed from July 12 to 16
during the transition.
Please enter your details below
Please enter your details below
Join us LIVE for a Q&A session on the latest iCS-digital™ PSC upgrade.
Find out what it means for you and ask all your questions
Juline VINCENT our R&D Project Manager and Digital PCR expert will answer you LIVE.
SAVE THE DATE: Thursday, November 7, 6pm CET/9am PDT/12 pm EDT.
Join us LIVE for our webinar!
Best Practices: Making your hPSC Quality Control workflow more efficient
Join Juline VINCENT, our R&D Project Manager, as she presents efficient quality control strategies specifically designed to address the unique characteristics of pluripotent stem cells.
Thursday, October 23, 12:00 PM EST / 6:00 PM CET
Please enter your e-mail address.