As cell therapy progresses, so does the required level of quality controls. Many of our clients need to justify the performance of the assays they use to certification and control authorities. Whether you have reached that stage or not, we believe it is always a good idea to seek reassurance in a solution that impacts the quality of your research work. So here it is for our iCS-digital™ PSC, broken down by key recommended criteria taken from the ICH Q2 scientific guidelines.
- 1. Precision: with repeatability of 7.37% and intermediate precision of 17.9% (versus the advisable ≤25%), you can rest assured that the results you get from our test will remain the same, no matter which operator performs it and no matter how many times you redo the test.
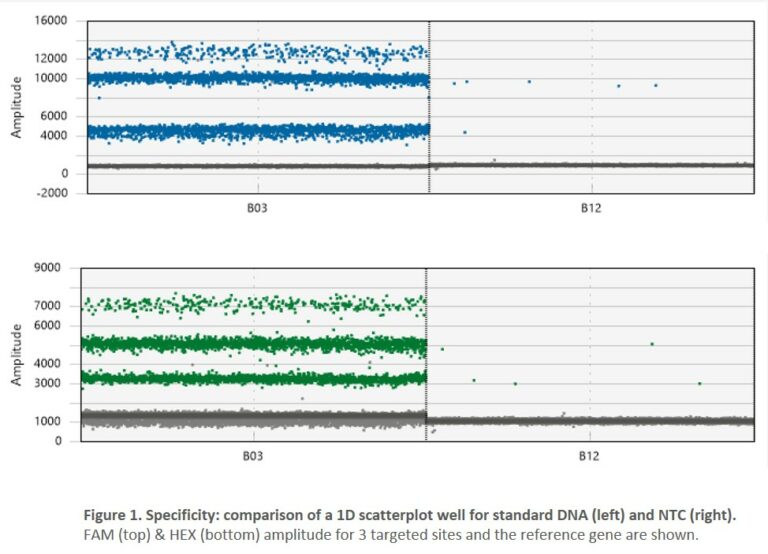
- 2. Specificity: digital PCR is a reliable tool when it comes to the detection of true positives. Our iCS-digital™ PSC false-positive rate is 0.098%, leaving very little room for error. It is well below the ≤5% usually expected for digital PCR-based assays.
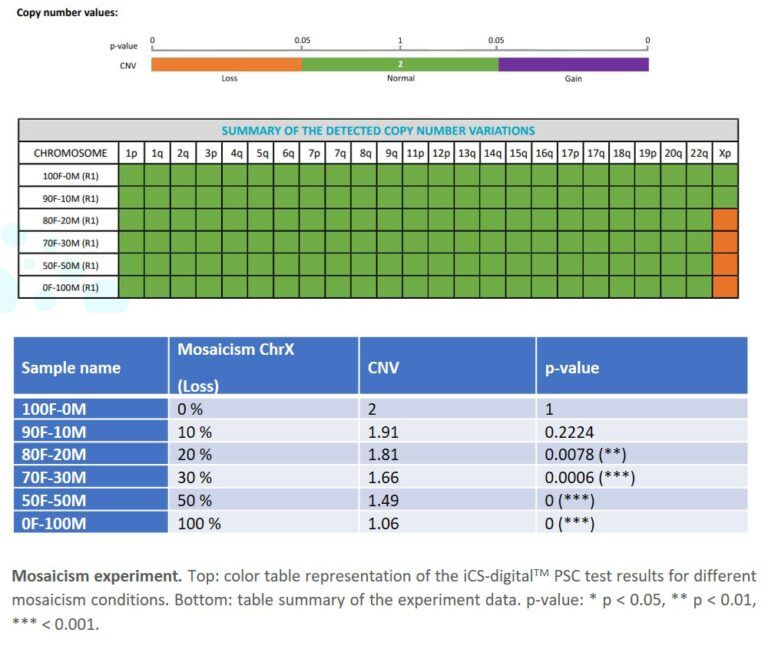
- 3. Sensitivity: the iCS-digital™ PSC achieves a 20% mosaicism detection rate, ensuring you pick up CNVs at early stages of your PSC workflow. This is a critical point for most PSC research scientists as abnormalities can take over a culture in as little as 5 passages (Andrews, 2021; Assou et al., 2020; McIntire et al., 2020). The 95% LOD corroborates this fact at a DNA concentration level.
- 4. Small DNA samples required: For our iCS-digital™ PSC 24-probe test, evaluations have shown that we can perform accurate and reliable analyses at 20% mosaicism from as little as 15 ng of DNA concentration. This means our clients don’t need to produce large numbers of cells for the purpose of QC quotas, which would incur additional costs and delays in their workflow. Instead, our clients can test for genomic stability more often, whilst keeping their lead times and budget in check.
For fully detailed facts and figures, download the detailed analytical performance sheet.
Email
LinkedIn
X